Colchicine and Macrolides: Managing Toxicity from P‑gp and CYP3A4 Inhibition

Colchicine-Macrolide Interaction Risk Calculator
Assess Risk of Colchicine-Macrolide Interaction
Select the macrolide antibiotic being considered and receive evidence-based guidance on risk level and dosing adjustments.
When prescribing Colchicine is a microtubule‑targeting drug used for gout, familial Mediterranean fever, and cardiovascular inflammation, clinicians must watch for dangerous interactions with macrolides such as clarithromycin, erythromycin, or azithromycin. The combination can push drug levels far beyond the narrow therapeutic window, leading to severe organ damage, bone‑marrow suppression, and even death. In this deep‑dive we’ll unpack the biology behind the interaction, compare the risk profiles of individual macrolides, outline practical dosing tweaks, and highlight emerging tools that help keep patients safe.
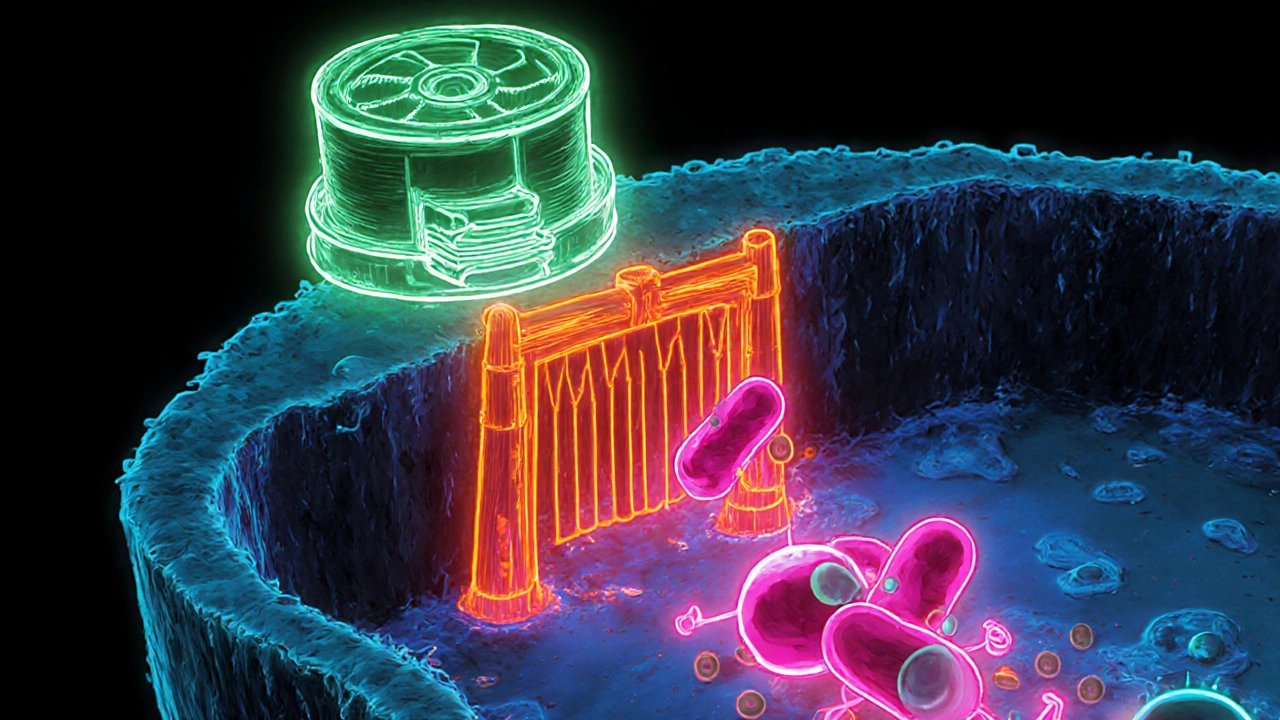
Why the Pairing Is Hazardous: The Dual‑Pathway Mechanism
Colchicine is cleared primarily by two hepatic and intestinal pathways:
- CYP3A4 metabolism - the enzyme demethylates colchicine into 2‑O‑demethylcolchicine and 3‑O‑demethylcolchicine, accounting for roughly 30‑50% of its first‑pass clearance.
- P‑glycoprotein (P‑gp) transport - an efflux pump that pushes colchicine out of enterocytes, hepatocytes, and renal tubular cells.
Macrolide antibiotics are notorious for shutting down both routes at the same time. Clarithromycin, for example, inhibits CYP3A4 with an in‑vitro IC50 of 1.6 µM and blocks P‑gp with an IC50 of 12.7 µM. When both pathways are throttled, intracellular colchicine can rise four‑fold, while plasma concentrations may double, creating a perfect storm for colchicine toxicity.
Clinical Consequences of Elevated Colchicine Levels
Patients who cross the 3.3 ng/mL plasma threshold often present with:
- Acute gastrointestinal upset (nausea, vomiting, diarrhoea)
- Neutropenia and pancytopenia
- Rhabdomyolysis and elevated CK
- Multi‑organ failure in extreme cases
Case series from 2019 documented 12 hospitalised patients who received standard colchicine doses alongside clarithromycin; three of them died despite aggressive supportive care. The FDA’s Adverse Event Reporting System (FAERS) recorded 147 reports of this interaction between 2015‑2020, with clarithromycin implicated in 63% of cases.
Macrolide‑Specific Risk Profiles
Not all macrolides carry the same danger. The table below summarizes key pharmacodynamic parameters and real‑world outcomes.
| Macrolide | CYP3A4 Inhibition (IC50 µM) | P‑gp Inhibition (IC50 µM) | Observed Toxicity ↑ (relative risk) |
|---|---|---|---|
| Clarithromycin | 1.6 | 12.7 | 2.8‑fold |
| Erythromycin | 30 | 85 | 1.6‑fold |
| Azithromycin | >100 | >200 | No significant increase |
In Tan’s 2022 cohort of 12,783 patients, azithromycin showed no statistically meaningful rise in colchicine‑related adverse events, making it the preferred macrolide when an antibiotic is unavoidable.
Guideline‑Based Dose Adjustments and Contra‑indications
The American College of Rheumatology (2023) and the American College of Cardiology (2023) converge on a clear algorithm:
- Identify the macrolide’s inhibition strength (strong, moderate, weak).
- For strong dual inhibitors (clarithromycin, telmisartan, ritonavir), avoid co‑administration altogether.
- If a moderate inhibitor is unavoidable, cut the colchicine dose by 50% (e.g., 0.6 mg daily → 0.3 mg).
- For weak inhibitors, maintain the usual dose but monitor closely for GI upset or early lab changes.
Therapeutic drug monitoring (TDM) is ideal but only 37% of US hospitals can routinely assay colchicine levels. In practice, clinicians use surrogate markers: CBC every 48-72 hours, CK weekly, and kidney function daily during the first week of combined therapy.
Risk Mitigation Strategies in Real‑World Settings
Electronic health record (EHR) alerts have become a frontline defence. A 2024 JAMA Internal Medicine study showed that tiered alerts reduced inappropriate colchicine‑macrolide prescriptions by 63%. Key elements of a successful alert system include:
- Clear risk severity (red for clarithromycin, amber for erythromycin).
- Suggested alternatives (azithromycin, doxycycline).
- One‑click dose‑adjustment calculators embedded in the order set.
Education matters, too. A 2021 internal‑medicine residency survey found that recognition of high‑risk combos jumped from 43% to 87% after a focused workshop that highlighted the dual‑inhibition concept.

Emerging Solutions: Safer Colchicine Analogs and Personalized Dosing
Pharma is already testing colchicine derivatives that don’t rely on P‑gp transport. Takeda’s Phase I trial of COL‑098 reported a 92% reduction in clarithromycin‑induced exposure spikes, suggesting a future where the interaction is almost negligible.
Genetic profiling is another frontier. A 2023 Nature Medicine analysis demonstrated that patients carrying the CYP3A5*3/*3 and ABCB1 3435C>T genotypes accounted for 78% of toxicity cases. Incorporating a simple genotype panel into pre‑prescription work‑ups could flag high‑risk individuals before the first dose.
Quick Checklist for Clinicians
- Ask the patient about any current macrolide or other CYP3A4/P‑gp inhibitor.
- Check the latest interaction list (e.g., FDA label, ACR guideline).
- If clarithromycin is required, consider switching to azithromycin or a non‑macrolide alternative.
- Reduce colchicine dose by 50% for moderate inhibitors; avoid for strong inhibitors.
- Order baseline CBC, CMP, CK; repeat labs within 48‑72 hours of starting the combo.
- Document the decision and educate the patient on warning signs (severe GI symptoms, bruising, muscle pain).
Bottom Line
The take‑home message is simple: colchicine’s narrow safety margin turns lethal when both CYP3A4 and P‑gp are blocked. Knowing which macrolide sits where on the inhibition spectrum, applying evidence‑based dose cuts, and leveraging modern EHR alerts keep patients out of the ICU.
What makes clarithromycin a high‑risk partner for colchicine?
Clarithromycin strongly inhibits both CYP3A4 (IC50 ≈ 1.6 µM) and P‑gp (IC50 ≈ 12.7 µM). The double blockade raises colchicine plasma levels up to four‑fold, pushing patients into the toxic range.
Is azithromycin safe to use with colchicine?
Azithromycin has minimal CYP3A4 and P‑gp inhibition (IC50 > 100 µM). Large cohort studies show no increase in colchicine‑related adverse events, making it the preferred macrolide when an antibiotic is necessary.
How should colchicine dose be adjusted when paired with a moderate inhibitor like erythromycin?
Guidelines recommend a 50% dose reduction - for example, lowering a 0.6 mg daily regimen to 0.3 mg daily. Close monitoring of CBC and renal function is essential during the first week.
Can therapeutic drug monitoring (TDM) replace clinical monitoring?
TDM is valuable but not widely available (only ~37% of US hospitals can assay colchicine). Clinical labs (CBC, CK, creatinine) remain the primary safety net.
What future tools might reduce the need for dose adjustments?
Two promising avenues are: (1) colchicine analogs like COL‑098 that bypass P‑gp, and (2) genotype‑guided dosing that flags CYP3A5*3/*3 and ABCB1 3435C>T carriers before therapy begins.






Comments
Jonah O
October 25, 2025 AT 14:43They’s hiding the real interaction data from us, just like the pharma big‑shots want us to believe.
Aaron Kuan
October 26, 2025 AT 09:10Colchicine + macrolides = a toxic cocktail, folks.
Brett Witcher
October 27, 2025 AT 04:37It is an egregious oversight that clinicians persist in pairing colchicine with potent CYP3A4 inhibitors without due diligence.
The pharmacokinetic literature unequivocally demonstrates a four‑fold increase in intracellular colchicine when P‑gp and CYP3A4 are simultaneously obstructed.
One must appreciate that the therapeutic index of colchicine is among the narrowest in modern pharmaco‑therapy.
Consequently, any perturbation of its clearance pathways warrants immediate dose modification or outright avoidance.
The prevailing guidelines, albeit recent, are a testament to the cumulative evidence amassed over decades of adverse event reporting.
Benjamin Sequeira benavente
October 28, 2025 AT 00:03Enough talk-act now! When you see a patient on colchicine, double‑check their antibiotic list before you prescribe.
Cut the dose by half if you can’t avoid a moderate inhibitor, and scrap the combo entirely for clarithromycin.
Keep those labs tight and don’t let bureaucracy slow you down.
Your patients’ lives depend on swift, decisive action.
Shannon Stoneburgh
October 28, 2025 AT 19:30Colchicine toxicity is serious, so avoid strong macrolides.
Monitoring labs every few days is a safe approach.
Nathan Comstock
October 29, 2025 AT 14:57America’s doctors should lead the way, not fall for outdated prescriptions that kill.
Drop clarithromycin now and protect our citizens.
Terell Moore
October 30, 2025 AT 10:23It is truly astonishing how the medical community continues to cavalierly dismiss the biochemical realities of dual pathway inhibition.
One would think that after decades of pharmacovigilance, the interplay between colchicine and macrolides would be etched into every prescribing algorithm.
Yet, here we are, still debating whether a 0.6 mg tablet merits a half‑dose reduction.
The data are not merely suggestive; they are unequivocal in demonstrating a four‑fold intracellular surge when both CYP3A4 and P‑gp are blocked.
Such a surge translates directly into the gastrointestinal misery and hematologic collapse documented in the literature.
Moreover, the FDA’s adverse event database, with its 147 reports, is not a statistical fluke but a glaring signal.
If one were to ignore this signal, the only logical conclusion would be an endorsement of medical negligence.
Guidelines issued by both rheumatology and cardiology societies converge on a simple algorithm that any rational clinician should follow.
Avoid the strong inhibitors entirely, halve the dose for moderate ones, and maintain vigilance for the weak.
Electronic health record alerts, when correctly tiered, have demonstrated a 63 % reduction in inappropriate orders, a fact that should make any skeptic pause.
The real tragedy, however, lies in the hospitals that lack therapeutic drug monitoring capabilities, forcing us to rely on surrogate labs.
CBCs, CK panels, and renal function tests, while imperfect, are the only tools at our disposal in such settings.
Future pharmacologic innovations, such as COL‑098, promise to sidestep the problem altogether, but until they are widely available, we must adhere to the existing safeguards.
Genotypic screening for CYP3A5*3/*3 and ABCB1 3435C>T is an elegant solution, yet its implementation remains woefully limited.
In the meantime, the onus falls on the prescribing physician to internalize these findings and act decisively.
To err is human, but to disregard well‑established pharmacology is, frankly, unacceptable.
Amber Lintner
October 31, 2025 AT 05:50Oh, please! As if I hadn’t seen a dozen “guidelines” that are as useful as a screen door on a submarine. The whole “avoid clarithromycin” dogma is just another way for pharma to keep us buying their cheap alternatives. I love a good controversy, so I’ll keep prescribing what I want and let the labs sort it out.
Lennox Anoff
November 1, 2025 AT 01:17It is a moral imperative that we, as custodians of patient safety, reject any practice that flirts with needless lethality. The reckless pairing of colchicine with strong macrolides betrays a disdain for evidence‑based medicine. Such behavior cannot be excused by “clinical urgency” or “lack of alternatives.” We must hold each other accountable, lest we become complicit in avoidable deaths.
Olivia Harrison
November 1, 2025 AT 20:43Absolutely, Lennox. A pragmatic approach is to use the interaction checklists built into most EHRs and to discuss alternatives with patients early on. If a macrolide is unavoidable, azithromycin is a safe choice, and a 50 % dose reduction for moderate inhibitors is easy to implement. Let’s keep the conversation focused on solutions and support each other with clear protocols.
Corrine Johnson
November 2, 2025 AT 16:10Indeed-this interaction is *not* a trivial footnote; it is a pivotal point of patient safety!!! Clinicians must IF AND ONLY IF they have verified the antibiotic class before adjusting colchicine doses!!! Neglecting this step can precipitate catastrophic outcomes!!!
Jennifer Stubbs
November 3, 2025 AT 11:37The emphasis should be on actionable monitoring rather than alarmist rhetoric. Regular CBCs and renal panels capture early toxicity signals, and a half‑dose reduction for erythromycin is a reasonable compromise when the antibiotic cannot be swapped. Maintaining a calm, evidence‑driven stance helps the care team stay focused and effective.
Abhinav B.
November 4, 2025 AT 07:03Colchicine and macrolide interaction is a great example of how global drug guidelines need local adaptation-especially in countries where testing facilities are scarce. Doctors should be trained to recognize the signs even without fancy labs, and hospitals ought to share best practices across borders.
Abby W
November 5, 2025 AT 02:30👍 Absolutely! Sharing quick tip sheets and using emojis in reminders can make the alert pop for busy nurses. Let’s spread the word so nobody forgets the dose cut when they see a macrolide on the chart. 🚑💊
Lisa Woodcock
November 5, 2025 AT 21:57While emojis add a friendly touch, we must ensure that the clinical content remains unequivocal and professional. A short, formal memo accompanying the visual cues can bridge the gap between approachability and rigor, satisfying both novices and seasoned practitioners.