Annual Savings from FDA Generic Drug Approvals: Year-by-Year Breakdown

Every year, the U.S. health system saves tens of billions of dollars simply because generic drugs hit the market. These aren’t hypothetical numbers. They’re real dollars pulled out of patients’ pockets, insurance bills, and government programs because a cheaper version of a brand-name drug became available. The FDA generic savings aren’t a slow, steady trickle-they spike in some years, dip in others, and the pattern tells a clear story about how drug pricing works in America.
What the Numbers Actually Mean
When you hear "savings from generic drugs," you need to know which number you’re looking at. There are two main ways to measure it, and they show very different sides of the same coin. The FDA tracks savings from new generic approvals-meaning the first time a generic version of a brand-name drug enters the market. They look at the 12 months after approval and calculate how much money was saved because the generic was cheaper. In 2019, that number hit $7.1 billion. That was the biggest single-year jump in over a decade. Why? Because three high-cost drugs-each used by hundreds of thousands of people-lost patent protection at once. One of them, a treatment for high cholesterol, dropped from $300 a month to $15. That’s not a small change. That’s life-changing for someone on a fixed income. But the FDA doesn’t count all generics. They only count the first-time entries. That’s where the Association for Accessible Medicines (AAM) comes in. They look at the entire market. In 2023, every single generic drug in use saved the U.S. health system $445 billion. That’s not just new approvals. It’s every pill, every injection, every inhaler that’s been on the market for years. The AAM number is the real total. It’s what keeps Medicare and Medicaid from going broke.The Year-by-Year Roller Coaster
If you look at the FDA’s first-generic savings year by year, it looks like a roller coaster:- 2018: $2.7 billion
- 2019: $7.1 billion
- 2020: $1.1 billion
- 2021: $1.37 billion
- 2022: $5.2 billion
Who’s Really Saving Money?
The $445 billion in total savings in 2023 didn’t just vanish into thin air. It went somewhere:- $206 billion saved in the commercial insurance market
- $137 billion saved by Medicare
- The rest by Medicaid and other public programs

Why Generic Prices Drop So Fast
When a brand-name drug loses exclusivity, the price doesn’t just dip. It collapses. The FDA says prices fall by more than 70% on average after the first generic enters. Why? Because competition is brutal. The first generic maker usually gets a small head start. But within months, three, five, even ten other companies jump in. Each one undercuts the last. By the time you get to the sixth generic, the drug might cost $10 a month. That’s not because the drug is cheap to make. It’s because the market is flooded. The FDA approved 742 generic applications in 2022 alone. That’s more than two per day. And they’re getting faster. Thanks to the Generic Drug User Fee Amendments (GDUFA), 95% of applications are reviewed in 10 months or less. That means generics hit the market quicker. And savings start sooner.Where the Savings Don’t Reach
Here’s the ugly truth: not all the savings make it to the patient. Pharmacists report that 92% of generic prescriptions are filled for under $20. The average copay is $6.97. That sounds great. But behind the scenes, pharmacy benefit managers (PBMs) negotiate rebates with drugmakers. In many cases, the PBM keeps a big chunk of that rebate instead of passing it to the patient. A 2023 Senate investigation found only 50-70% of generic savings actually reach consumers. That’s why some people still pay hundreds for a generic drug. It’s not the price of the pill. It’s the system. Medicaid programs, like California’s Medi-Cal, have seen $23 billion in savings. But patients in those programs still face high out-of-pocket costs because of how rebates are structured.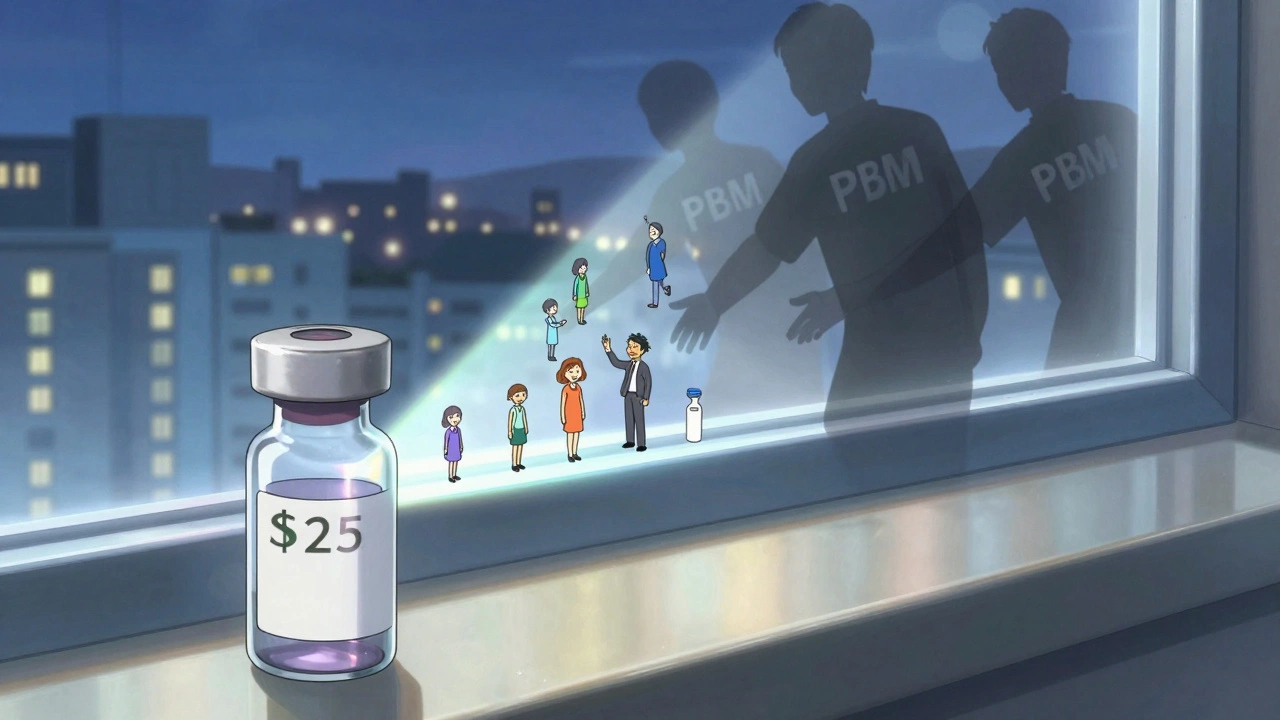
What’s Next? Biosimilars and Complex Drugs
The easy wins are mostly behind us. The big, simple drugs-like statins, antibiotics, and blood pressure meds-already have generics. The next wave is harder: complex drugs, injectables, and biologics. Biosimilars-generic versions of biologic drugs-are slowly entering the market. As of August 2024, the FDA had approved 59 of them. They’re not as cheap as traditional generics. A biosimilar might be 15-35% cheaper, not 90%. But they’re still saving money. The AAM projects that by 2028, total savings from generics and biosimilars combined will hit $3.9 trillion since 2014. The FDA is working on new rules to speed up approvals for complex generics. That’s good news. But brand companies are fighting back. They use legal tricks, patent extensions, and safety restrictions (called REMS) to delay generics. The FDA’s 2023 Drug Competition Action Plan is trying to shut those loopholes down.The Bigger Picture
Generics make up 90% of all prescriptions in the U.S. But they account for just 13% of total drug spending. That’s the power of competition. Without generics, the U.S. health system would be unaffordable. The numbers don’t lie. In 2022 alone, new generic approvals saved the system $5.2 billion in the first year. The total savings from all generics? $445 billion. That’s enough to cover the entire health care budget of a state like Texas for a year. The challenge isn’t finding more generics. It’s making sure the savings reach the people who need them. Until rebates are transparent and PBMs are held accountable, patients will keep paying more than they should-even for a $10 pill.How much do generic drugs save the U.S. each year?
In 2023, generic and biosimilar drugs saved the U.S. health system $445 billion, according to the Association for Accessible Medicines. This includes savings from all generics in use, not just new approvals. The FDA reports additional savings from new generic approvals each year-$5.2 billion in 2022 alone from first-time entries.
Why do generic drug savings vary so much year to year?
Savings spike when high-cost brand-name drugs lose patent protection. In 2019, three major drugs went generic at once, generating $7.1 billion in savings. In 2020, only low-revenue drugs came off patent, so savings dropped to $1.1 billion. It’s not a steady flow-it’s tied to which drugs lose exclusivity, making it unpredictable.
Do patients actually see lower prices when generics are approved?
Sometimes, but not always. While generic drugs often cost under $20 per prescription, pharmacy benefit managers (PBMs) sometimes keep rebates instead of passing them to patients. A 2023 Senate report found only 50-70% of generic savings reach consumers directly. So a drug might drop from $300 to $25, but a patient’s copay might only fall from $100 to $75.
What’s the difference between FDA and AAM savings numbers?
The FDA measures savings only from new generic approvals during their first 12 months on the market. The AAM measures total savings from every generic drug used in a calendar year. The FDA number is about new competition; the AAM number is about total market impact. The AAM number is much larger because it includes decades of accumulated savings.
Are biosimilars saving as much as traditional generics?
Not yet. Traditional generics can cut prices by 80-90%. Biosimilars-generic versions of complex biologic drugs-are typically 15-35% cheaper. As of August 2024, the FDA had approved 59 biosimilars. While their savings are growing, they still represent a small fraction of total generic savings. Their impact is expected to rise as more biologics lose patent protection.
Which therapeutic areas benefit the most from generic drugs?
Heart disease drugs saved patients $118 billion in 2023. Mental health medications saved $76 billion. Cancer treatments saved $25 billion. These are the areas where drug prices were highest and patient demand was greatest. Generics made these treatments affordable for millions who otherwise couldn’t afford them.
How many generic drugs are approved each year?
The FDA approved 742 generic drug applications in 2022. In 2021, it was 633. These numbers include both first-time generics and additional versions of drugs already available as generics. The number of first-time generic approvals varies yearly-from 48 in 2021 to 75 in 2022-depending on patent expirations.
Why do some generic drugs still cost a lot?
Some generics cost more because they’re complex to manufacture, like injectables or inhalers. Others are priced high because there’s little competition-only one or two makers. The FDA is working to speed up approvals for these complex drugs. Also, some brand companies use legal tactics to delay generic entry, keeping prices high longer.






Comments
Gareth Storer
December 6, 2025 AT 02:11So let me get this straight - we’re celebrating a $445 billion savings because Big Pharma finally let go of some drugs they’d been milking for decades? And you call that a win? Meanwhile, my cousin still pays $120 for a 30-day supply of metformin because her PBM ‘negotiated’ the rebate into a vacation fund. This isn’t savings - it’s theater with a balance sheet.
Pavan Kankala
December 7, 2025 AT 08:28They don’t want you to know this, but the FDA is basically a puppet of the pharmaceutical lobby. Every time a generic gets approved, it’s because the brand company already sold the patent rights to a shell corporation overseas - then they ‘license’ it back to the generic maker at 10x the cost. The ‘savings’? A mirage. The real money flows through offshore accounts, tax havens, and private equity firms that own both the brand AND the generic. They’re playing 4D chess while we’re stuck counting pills.
Martyn Stuart
December 8, 2025 AT 20:48Let’s be clear: the FDA’s $5.2 billion in 2022 savings? That’s just the tip of the iceberg. The AAM’s $445 billion? That’s the whole iceberg - and it’s melting because of competition. But here’s the thing: the real hero isn’t the FDA - it’s the generic manufacturers who risked billions to reverse-engineer drugs, navigate regulatory hell, and undercut monopolies. Without them, insulin would still be $400 a vial. And yes - PBMs are crooked - but that’s a separate, systemic failure. Don’t blame the generics for the greed of middlemen.
Jessica Baydowicz
December 9, 2025 AT 19:21OMG I just cried reading this - like, actual tears. Imagine being able to afford your anxiety meds for $6 instead of $150? That’s not a drug - that’s freedom. And the fact that heart meds saved $118 BILLION? That’s not a statistic - that’s a million people breathing easier. We need to scream this from the rooftops. Generics aren’t ‘cheap’ - they’re courageous. And we owe them SO MUCH.
Shofner Lehto
December 11, 2025 AT 06:58The real scandal isn’t the savings - it’s how slow the FDA is to approve complex generics. They approved 742 applications in 2022? That’s impressive - but half of those were repeat versions of already-approved drugs. The real bottleneck is the injectables, inhalers, and biosimilars. And until the FDA cracks down on REMS abuse and patent evergreening, we’re just rearranging deck chairs on the Titanic. The system’s rigged - and the fix isn’t more approvals. It’s more guts.
Emmanuel Peter
December 12, 2025 AT 01:31Everyone’s acting like this is some heroic victory. Nah. The only reason prices dropped is because the brand companies were already planning to sunset those drugs anyway. They let generics in because they’d already shifted profits to the next miracle drug. The real winners? The investors who bought the brand company’s stock before the patent cliff and sold after the generic hit. Meanwhile, you’re still paying $20 for a pill because your insurance company doesn’t care - they just want the rebate.
Ollie Newland
December 13, 2025 AT 16:38Let’s unpack the biosimilars thing - they’re not just ‘expensive generics.’ Biologics are living molecules, not chemical formulas. Manufacturing them is like cloning a snowflake - no two batches are identical. So yeah, 15-35% savings sounds small, but it’s massive when you’re talking about $50,000/year drugs. The FDA’s 59 approvals? That’s the thin edge of the wedge. In five years, we’ll see 10x that. And when biosimilars hit the oncology market hard? That’s when the real revolution starts.
Rebecca Braatz
December 15, 2025 AT 13:06Thank you for this breakdown. Seriously. I work in community health, and every day I see people choosing between insulin and groceries. This isn’t policy - it’s survival. The $445 billion? That’s not just a number - it’s a thousand grandmas breathing without a nebulizer, a hundred thousand kids with asthma getting their inhalers, and a million people with depression finally able to afford their meds. Let’s not just celebrate the savings - let’s demand they reach the people who need them. No more middlemen. No more rebates. Just fair prices. Period.